
Some additional relationships that might come up are briefly noted in Section F.Į.
#Organic chemical equation balancer free
Thus, we are free to write whichever of them seems most chemically appropriate for a particular half-cell we can then combine half-cell equations no matter which electron form they show by using these relationships. Overall, Equations 7, 11 and 15 allow us to interconvert among three formal ways of representing electron intermediates: free electrons or hydrogen or oxygen atoms. Another relationship we might have used is: Eqn 11 allowed us to combine those into a single equation - in this case, one with all electron forms canceling out. In that case, we had two partial reactions, one written with \(e^-\) and one written with H. We used this relationship in Section B.7. Eqn 7 allowed us to combine those into an equation showing only one electron form.Įqn 11 is a relationship between H + and e. In that case, we had two partial reactions, one showing H and one showing O. We used this relationship in Section B.4. We used two such relationships above:Įqn 7 is a relationship between H and O atoms: To do that, we recognize various relationships between them, all of which follow good chemical logic. In combining the half cells, we need to eliminate these formal intermediates. That is, redox half cells may show the formal species e-, H or O. However, the half-cell reactions are instructive to us, as well as useful in working out the complete equation.įor simple chemicals, we may write the redox half cell with free electrons, making use of the oxidation numbers. These unrealistic species must disappear when we combine the two half cells into an equation for the complete redox reaction. These “half-cell reactions” are hypothetical, and we often write them with unrealistic species, such as free electrons or hydrogen atoms. We recognize a reduction half cell because there is a gain of electrons - or a gain of hydrogen or loss of oxygen. We recognize an oxidation half cell because there is a loss of electrons equivalently, there may be a loss of hydrogen or gain of oxygen. To help us visualize and balance redox reactions, we commonly divide them into two parts: one for oxidation and one for reduction. Redox reactions involve electron transfer However, free electrons and free hydrogen atoms generally do not belong in final equations for real processes. The choice of which species are acceptable depends on the specifics of the case at hand.

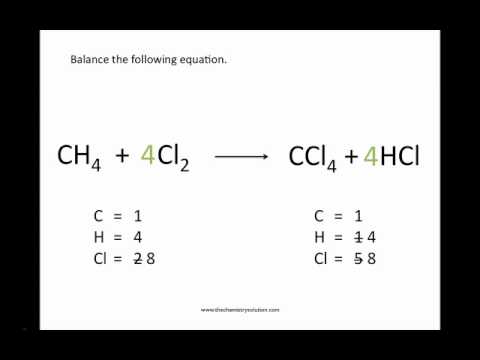
That is fine here, since the reaction occurs in acidic aqueous medium. The result is Eqn 1 - the final desired equation.ģ CH 3CH 2OH + 2 Cr 2O 7 2 - + 16H + → 3 CH 3COOH + 4 Cr 3 + + 11 H 2O (1) To get the electrons to balance out when the two are combined, we take 2 times Eqn 2, and add 3 times Eqn 12. The form of the oxidation half cell that is convenient here is Eqn 12 it shows 4 e. The reduction half cell is Eqn 2, and shows 6 e. To obtain the final equation for the overall reaction, we combine the reduction half cell and the oxidation half cell, as usual with redox equations.
To do that, we re-write the H atom, H, as the sum of its parts:ĬH 3CH 2OH + H 2O → CH 3COOH + 4 H + + 4 e - (12) To combine these two half-cell equations, we need to get them in the same electron language.
The former shows electron transfer in terms of free electrons the latter shows electron transfer in terms of hydrogen atoms. The oxidation half cell is described in Eqn 9. The reduction half cell is described in Eqn 2. We now have equations for two half-cell reactions. This is addressed below, in Section B.6.) (In practice, many people will be comfortable balancing the complete oxidation half-cell equation without considering the steps. I think it will be useful here to look at the two steps separately, since they are different in the H and O issues. The reaction being considered is actually the sum of two distinct reactions. If we are considering a C atom getting oxidized (losing electrons), showing loss of an H atom is chemically logical, but showing loss of an H+ ion is not. It is important to understand that, because oxidation and reduction deal with electron transfers. That statement refers to O and H atoms, just as the symbols indicate, not to ions. Oxidation is the addition of O or removal of H.
#Organic chemical equation balancer how to
For the record, I show how to balance this equation using ON in Note 1.) Thus in organic chemistry, we often deal with oxidation and reduction of C by looking at changes in the number of H and O. True, but they often are difficult with complex organic compounds. (An alert reader may counter that the ON in this particular example are actually not difficult at all. Dealing with ON for carbon often is difficult. Let’s look at this half reaction more carefully, trying to use good organic chemistry. The oxidation half reaction is the oxidation of ethanol to ethanoic acid.


 0 kommentar(er)
0 kommentar(er)
